
China is a big country in the production and consumption of skin care products. With the continuous development of the economy, the consumption level of Chinese residents is also increasing. The market size of skin care products has expanded significantly. Cosmetics have also changed from luxury goods to indispensable daily necessities, and industry competition has become increasingly fierce. fierce. At a time when new brands, new effects, and new ingredients are constantly emerging, consumers pay special attention to the safety of product ingredients and the authenticity of efficacy. In order to strengthen the management of the safety and efficacy of cosmetics, the State Food and Drug Administration requires cosmetics manufacturers to evaluate the efficacy claims and safety of cosmetics before the products are put on the market.
A series of policies such as various management measures, work norms, and technical guidelines to be implemented in 2021. Will there be any unclear test items during the product filing process? Not sure about the filing process? Frequent filing audit problems?
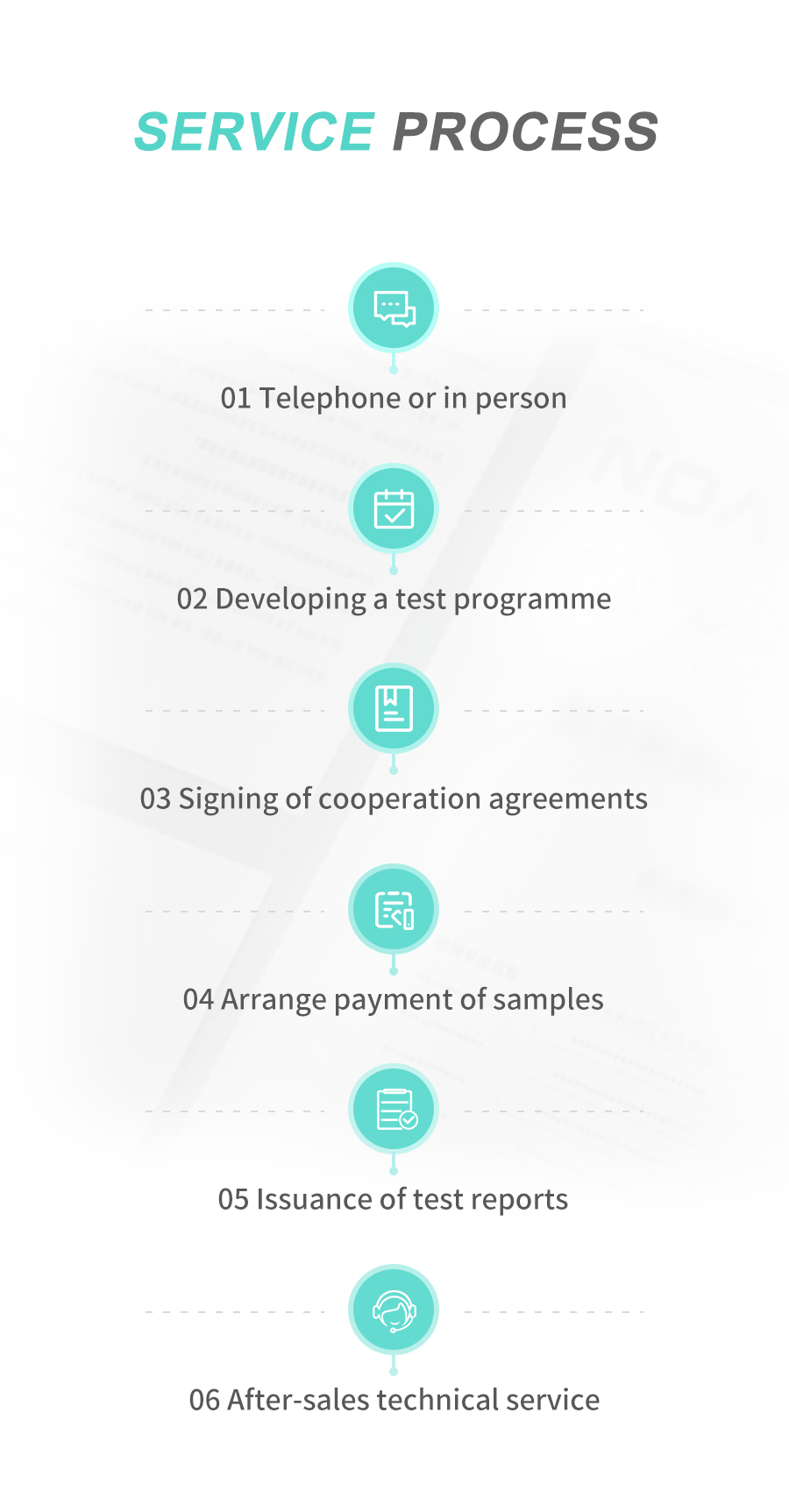
NOA has built a first-class testing platform around cosmetics, providing customers with product filing testing, formula design draft regulations review, product safety evaluation, efficacy evaluation project testing, system operation guidance, legal knowledge answers, etc. Customer assistance. Our one-stop solution can assist companies to produce satisfactory, safe and effective cosmetics, and provide customers with professional analysis, testing, testing, research and development, regulatory consultation and other technical services at any stage of product development and production.
| NO. | Efficacy Claims | Human Efficacy evaluation Test | Consumer Performance Test | Laboratory Test | Literature Data or Research Data |
| 1 | Freckle-removing and whitening① | √ | |||
| 2 | Sunscreen | √ | |||
| 3 | Anti-hair loss | √ | |||
| 4 | Acne-removing | √ | |||
| 5 | Nourishing② | √ | |||
| 6 | Repairing② | √ | |||
| 7 | Anti-wrinkle | * | * | * | △ |
| 8 | Tightening / Firming | * | * | * | △ |
| 9 | Relieving / Soothing | * | * | * | △ |
| 10 | Oil-control | * | * | * | △ |
| 11 | Exfoliating | * | * | * | △ |
| 12 | Hair breakage prevention | * | * | * | △ |
| 13 | Anti-dandruff | * | * | * | △ |
| 14 | Moisturizing | * | * | * | * |
| 15 | Hair-care | * | * | * | * |
| 16 | Specific claims (claims to be suitable for sensitive skins, be a tear-free formula) | * | * | ||
| 17 | Specific claims (ingredient efficacy) | * | * | * | * |
| 18 | Claims to be gentle (no stimulation) | * | * | * | △ |
| 19 | Claims of quantitative indicators (time, statistical data, etc.) | * | * | * | △ |
| 20 | New efficacy | Select the appropriate evaluation basis according to the specific efficacy claims. | |||
Note:
1. Items drawn in the option bar are required;
2. Drawing * in the Options Bar is optional, but you must select at least one of it;
3. The △ is painted in the option bar, but it must be used together with the human efficacy evaluation test, consumer use test, or laboratory test.
Note:
① Only plays the effect of freckle whitening through physical covering, and clearly has a physical effect in the label, can be exempted from submitting the application of product efficacy claim evaluation materials;
② If the effect of action is only hair, can choose real hair in vitro for evaluation.
| Cosmetic Safety Testing | Cosmetics efficacy test | Consumer testing | Toothpaste efficacy test | laboratory test | In vitro substitution experiments |
| Skin closed patch test (24h) | whitening | crease resistant | White teeth | Dry combing (hair care) | In vitro chromosomal aberration assay in mammalian cells |
| Skin Closed Spot Stick Test (CMA) | Prevent take off | Compact | Reduce plaque | Wet combing (hair care) | In vitro gene mutation in mammalian cell assay |
| Skin closed patch test (48h) | remove acne | leisurely | Fresh breath | Gloss (hair care) | Ames test |
| Sensitive skin patch(24h) | servicing | oil-control | Maintain teeth and periodontal tissue (including gingival) health | Friction force (repair) | Cytotoxicity test |
| Light spot paste | crease resistant | Exfoliator | Keep your mouth healthy | Single hair stretch / toughness (hair resistant) | Cosmetic skin irritation test |
| Safety evaluation of human trial experiment | Compact | Prevent broken hair | Reduce tooth stains | Leave incense (fragrance) | Keratocyte assay of acute toxicity in cosmetics |
| Sensitive skin trial (questionnaire survey and screening) | leisurely | anti-dangdruff | Reduce soft scale | Anti-wrinkle effect | |
| Sensitive skin trial (questionnaire survey + lactic acid tingling test screening) | oil-control | Moisture | Inhibition of dental plaque | Compact effect | |
| Sensitive skin trial (questionnaire survey + lactic acid tingling test + TEWL screening) | Exfoliator | Hair care | Anti-dentin sensitive | leisurely | |
| Human skin open skin patch test | anti-dangdruff | Specific claims (declared sensitive skin, tear-free formula) | Remove stains and add white | anti-dangdruff | |
| Evaluation of Human Repetitive Injury Spot Tie Test (HRIPT) | Moisture | Specific claims (raw material efficacy) | Anti-dental stones | Antioxidant | |
| Evaluation of Human Repetitive Injury Spot Tie Test (HRIPT) | Makeup waterproof performance test | Say mild (no stimulating) | Reduce gingival inflammation | ||
| Acogenicity (Trial test) | Makeup durability test | Proclaim to quantitative indicators(Time, statistics, etc.) | |||
| Aclinicity (Trial test) | cleaning | ||||
| Tearless Formula (Trial Test) | take off formal dress and ornaments | ||||
| Beauty modification | |||||
| lose hair or feathers | |||||
| deodorization |


According to article 19 of the "Cosmetics efficacy Declaration Evaluation Code": the summary of the cosmetics efficacy declaration basis should briefly list the content of the product efficacy declaration basis, including at least the following information:
(1) Basic product information;
(2) Efficacy claims evaluation projects and evaluation institutions;
(3) A brief description of the evaluation methods and results;
(4) The conclusion of the efficacy claim evaluation shall clarify the relationship between the efficacy claim of the product and the evaluation method and results.










Tel:+86-400 821 5138
Fax:+86-21 3327 5843
Email:noa@noagroup.com